

It is corrosive in nature and categorized as an irritant.It is very harmful to the environment and a major health hazard.Chromium III Sulfate is an inorganic ionic compound.The molecular formula of Chromium III Sulfate is Cr 2(SO 4) 3.It serves as electrolyte to obtain chromium metal.It is used in chrome plating to decorate and protect metals.It is also used in green paints, ceramic glazes and inks.It is used in textile dyes particularly for dyeing of khaki cloth.It is extensively used in the leather industry during the process of tanning.It turns green when it is subjected to heat.Ĭhromium III Sulfate is produced as a byproduct in the process of Jones Oxidation during which a solution of sodium dichromate or potassium dichromate is dissolved with sulfuric acid to oxidize alcohols.Ĭhromium(III) sulfate can also be obtained by treating chromium III hydroxide with dilute sulfuric acid.
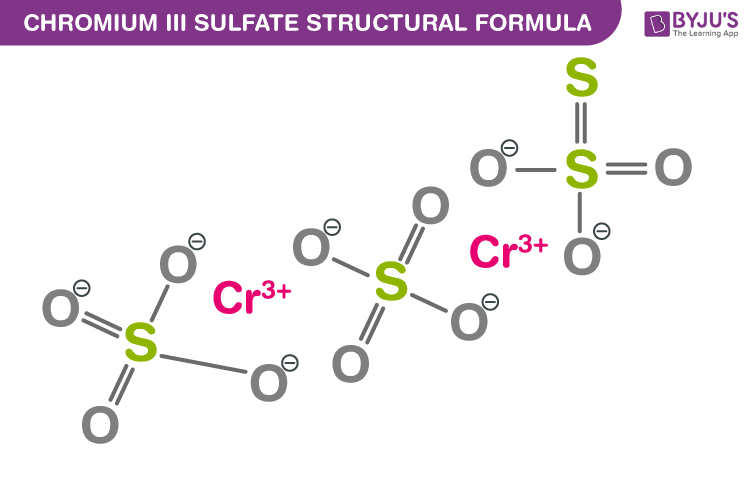
Chromium III sulfate is a blue-gray or violet-gray amorphous solid.When we write the two together we get Cr 2 (SO 4 ) 3. So, to arrive at the formula of Chromium III Sulfate, we will interchange the charges of ions Cr +3and SO 4 -2 to get Cr 2 and (SO 4 ) 3.Cr is placed outside the parentheses as it is not a polyatomic ion. So, polyatomic ion SO 4 -2 will be placed inside parenthesis. The sulfur and oxygen atoms share a covalent bond.Formula Molar mass of ion Number of ions Mass of ion in one mole of chromium (III) sulfide Cation Cr3+ g/mol mol g Anion 11 52- g/mol mol g/mol Molar mass chromium (III) sulfide. So, the Charge carried by the chromium cation Cr +3 will be written as the subscript of the sulfate anion SO 4 -2 and the Charge carried by the sulfate anion SO4-2 will be written as subscript for the chromium cation Cr +3. plete the table below for calculating the molar mass of the ionic compound chromium (III) sulfide.Chromium III has a valence of 3 and Sulfate has a valence of 2, we will need 2 chromium and 3 sulfate ions to balance 6 valence ties.It is an ionic compound where Chromium (Cr) is the metal with Cr+3 cation and sulfate is the SO4-2 anion. The formula of Chromium III Sulfate is Cr 2(SO 4) 3.The percentages of individual elements of Cr 2(SO 4) 3 (dichromium trisulfate) are Cr - 26.52%, O - 48.95% and S - 24.53%.As seen in the structure of Chromium III Sulfate in the image above, the ratio of chromium to sulfate in this inorganic compound is 2:3.


 0 kommentar(er)
0 kommentar(er)
